Introduction and Objectives:
The phase 3 A-LONG and Kids A-LONG studies demonstrated the safety and efficacy of rFVIIIFc for the control and prevention of bleeding episodes in subjects with severe hemophilia A. This subgroup analysis of A-LONG and Kids A-LONG examined bleeding frequency with rFVIIIFc prophylaxis in subjects with target joints at baseline.
Methods:
A-LONG (≥12 y) and Kids A-LONG (<12 y) subjects with a history of target joints (a major joint with ≥3 bleeding episodes in a 6-month period) who were previously treated with FVIII and received on-study rFVIIIFc prophylaxis were identified. Self-reported pre-study 12- month bleeding history and on-study annualized bleeding rate (ABR) for all bleeds were evaluated.
Results:
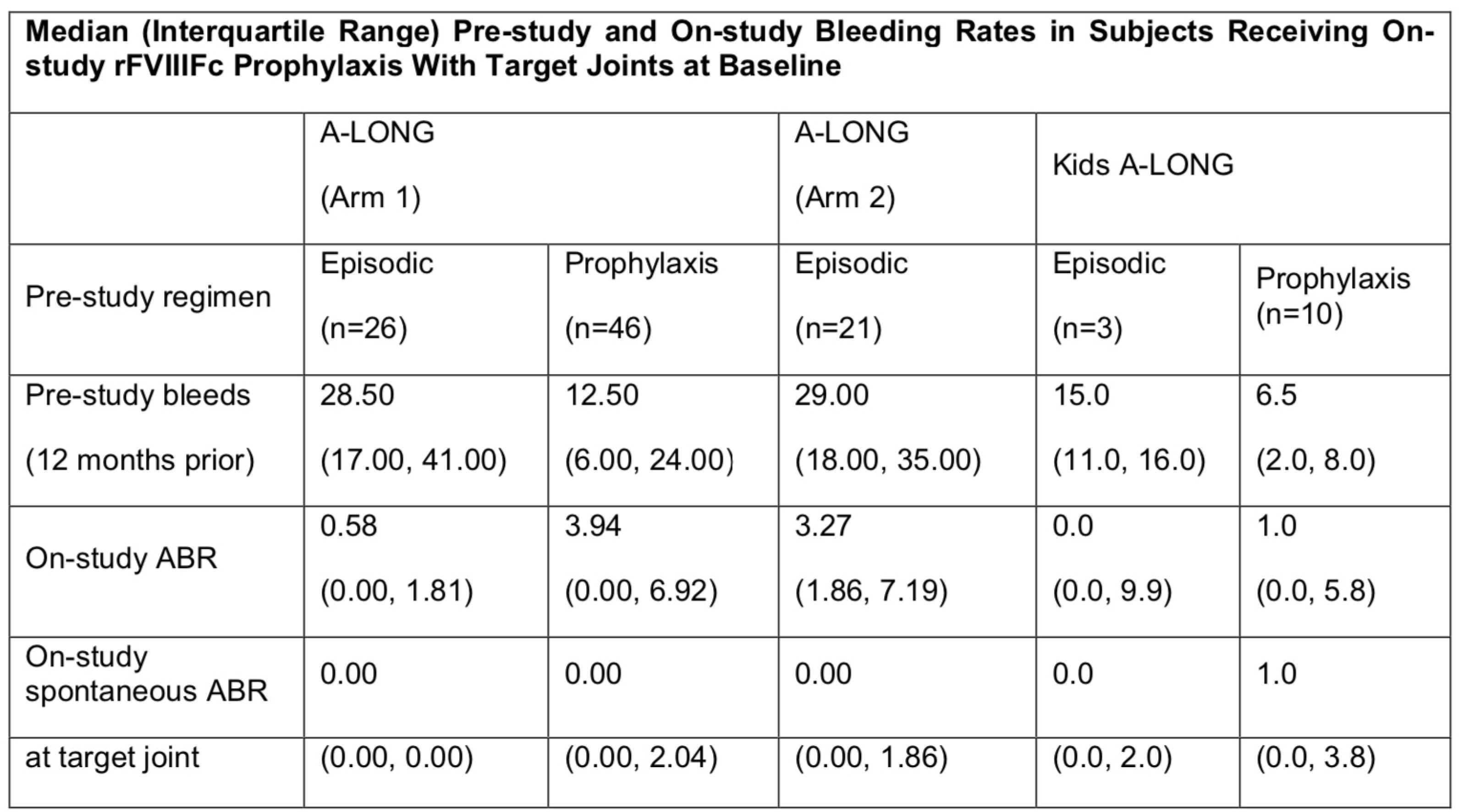
93/141 subjects in A-LONG and 13/69 subjects in Kids A-LONG had target joints at baseline. The most prevalent locations of pre-study target joints identified at baseline among subjects in A-LONG were the elbow (61.7%) and ankle (59.6%). Similarly, in Kids A-LONG, these were the ankle (69.2%) and elbow (30.8%). A total of 59.7% and 52.4% of subjects receiving individualized (Arm 1) and weekly (Arm 2) rFVIIIFc prophylaxis in A-LONG, respectively, and 53.8% of subjects receiving rFVIIIFc prophylaxis in Kids A-LONG with target joints at baseline had no target joint bleeding episodes on-study. Median on-study ABRs with rFVIIIFc prophylaxis tended to be lower than pre-study bleeding rates in subjects with target joints at baseline (Table).
Conclusions:
For subjects with severe hemophilia A who had target joints at baseline, rFVIIIFc prophylaxis lowered bleeding rates across age groups compared with pre-study FVIII treatment.